Ionisation
Atoms are comprised of a nucleus of protons and neutrons, which can be thought of as surrounded by a cloud of orbiting electrons. When one (or more) electron is stripped off or added to the atom, it is no longer electrically neutral and an ion is formed; the atom is said to be ionised. The energy required to ionise an atom can come from collisions or from interacting with electromagnetic radiation (photoionisation).
An example of ionisation in an astronomical context is that found in planetary nebulae and massive star-forming regions. These objects contain, amongst other gases, hydrogen and helium. The intense UV-radiation from the white dwarf contained in a planetary nebula or the OB association in a massive star-forming region photoionises the surrounding gas. When hydrogen and helium are ionised, they become HII and HeIII, respectively. The Roman numeral ‘II’ means that a single electron has been lost, while ‘III’ means that the atom has lost two electrons. Recombination line emission occurs when an ion recaptures an electron and emits a photon.
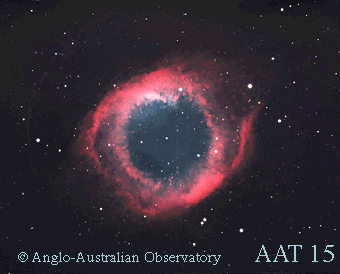
Credit: © 1993-2002, Anglo-Australian Observatory
Study Astronomy Online at Swinburne University
All material is © Swinburne University of Technology except where indicated.